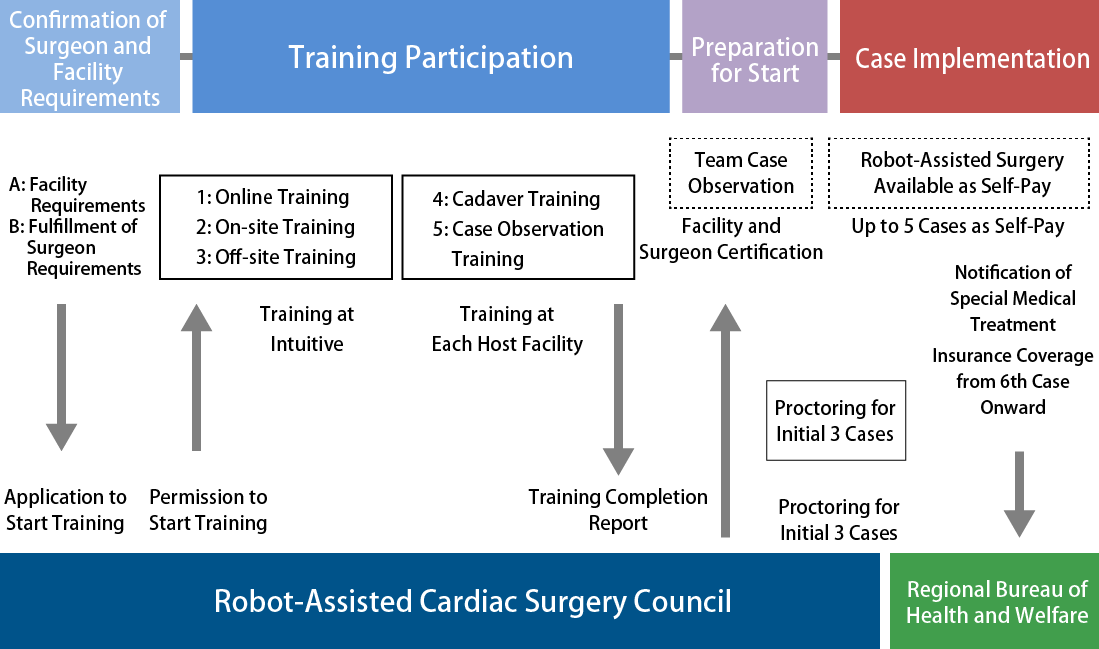
New Facility Certification and New Surgeon Certification
New Facility Certification
To ensure that a system capable of safely performing robot-assisted cardiac surgery is in place, the team must be experienced in right mini-thoracotomy cardiac surgery. The purpose of the review is also to assess whether the facility can consistently maintain a stable number of robot-assisted cardiac surgery cases. (Application must be submitted before training begins)
- At least two board-certified cardiovascular surgeons accredited by The Japanese Board of Cardiovascular Surgery (hereinafter referred to as "board-certified cardiovascular surgeons") must be employed full-time
- At least one cardiovascular surgery training facility instructor must be employed full-time
- At least one certified clinical perfusionist must be employed full-time
- At least one board-certified cardiovascular anesthesiologist must be employed full-time
- At least 100 surgeries using cardiopulmonary bypass must be performed annually
- At least 20 cases of minimally invasive cardiac surgery via right mini-thoracotomy (hereinafter referred to as "MICS") must have been performed within the 2 years prior to application
At least 15 of these cases must be mitral valve repair procedures performed via thoracoscopic valvuloplasty (procedure code K554-2)
- The applicant must have participated in the surgery in one of the following roles: supervisor, primary surgeon, or assistant
New Surgeon Certification
To assess the suitability of the applicant as a console surgeon for robot-assisted cardiac surgery. (Application must be submitted before training begins)
- The console surgeon must be a board-certified cardiovascular surgeon
- Must be proficient in video-assisted valvuloplasty via right mini-thoracotomy
Must have performed at least 20 MICS procedures as the primary surgeon within the 2 years prior to the application date
At least 15 of these cases must be mitral valve repair procedures performed via thoracoscopic valvuloplasty (procedure code K554-2)
In the fiscal year 2024 insurance revision, robot-assisted valve replacement was added to insurance coverage.
Since the basic technique in robot-assisted cardiac surgery is thoracoscopic valvuloplasty, thoracoscopic valve replacement experience is not included in the requirements for new facility certification and new surgeon certification.
Facility and surgeon certification based solely on robot-assisted valve replacement will not be granted.
Training Enrollment
Before starting training, submit documentation to the Robot-Assisted Cardiac Surgery Council (hereinafter referred to as "the Council") for review to verify that the facility standards and new surgeon standards are met.
Complete all required information on the Training Enrollment Condition Checklist and submit the following documents to the Council. At the same time, notify the Council via the contact form on this website that the documents have been sent.
Training Enrollment Condition Checklist [PDF file 180KB]
Required Documents
- Training Enrollment Condition Checklist
- Proof of Facility Standards
2-1.Copies of board certification for at least two board-certified cardiovascular surgeons
2-2.Copy of certification for at least one cardiovascular surgery training facility instructor
2-3.Copy of certification for at least one certified clinical perfusionist
2-4.Copy of certification for at least one board-certified cardiovascular anesthesiologist
2-5.List proving that at least 100 surgeries using cardiopulmonary bypass are performed annually
Must include surgery dates and procedures (personal information should be omitted)
2-6.Documentation proving the performance of at least 20 right mini-thoracotomy cardiac surgeries (surgical records)
Must include at least 15 thoracoscopic valvuloplasty procedures (procedure code K554-2)
- Documents Proving New Surgeon Standards
3-1.The console surgeon must be a board-certified cardiovascular surgeon accredited by The Japanese Board of Cardiovascular Surgery (copy of cardiovascular surgery board certification)
3-2.Surgical records and list proving that at least 20 MICS procedures have been performed as the primary surgeon
3-3.Surgical records and discharge summaries for all applicable cases to verify surgical experience and complications
3-4.Echocardiography findings at discharge for mitral valve repair cases
※Method for Submitting Application Documents
All application documents must be submitted to the secretariat by the applicant as PDF files on USB or similar media.
The Excel surgical list should be submitted in its original format without converting to PDF.
Important Matters Related to the 2024 Medical Fee Revision
※In the 2024 medical fee revision, robot-assisted valve replacement surgery was newly included as video-assisted valve replacement surgery (using endoscopic support devices). Since valvuloplasty is the global standard in robot-assisted cardiac surgery, the Council will continue to conduct facility and surgeon certification reviews based on MICS valvuloplasty experience for console surgeon certification.
※To begin robot-assisted valve replacement surgery, proctoring for robot-assisted valvuloplasty must be completed
For insurance-covered procedures, notification as a specified medical treatment fee is required.
※However, if conversion from valvuloplasty to valve replacement becomes necessary, it may be performed at the proctor's discretion, but insurance coverage will not apply if notification to the Regional Bureau of Health and Welfare has not been made.
Supplementary Notes on Surgeon Standards
- Right parasternal incision and partial sternotomy are not recognized as right mini-thoracotomy surgery. MIDCAB experience is also not recognized.
- Patient names must be anonymized by redaction or similar means. Assign the same sequential number to surgical records, discharge summaries, and echocardiography findings so they can be cross-referenced with the summary.
※The training enrollment criteria are intended for facilities and surgeons who are proficient in MICS surgery and wish to proceed to robot-assisted surgery. Even if the number of cases and other conditions are met, the application may be suspended if it is determined that robot-assisted surgery cannot be performed safely. In such cases, the surgeon may reapply after gaining the specified additional experience.
※The review standard is the ability to perform mitral valve repair (standalone) using standalone MICS within approximately 120 minutes of aortic cross-clamping. For combined surgeries, the evaluation will take into account the time required for other procedures.
※False applications will not be accepted for 3 years.
Application for Implementing Facility After Training Completion
After completing training, submit a training completion report to the Council and apply for implementing facility status. The Council will officially issue an implementing facility certification. After being recognized as an implementing facility, da Vinci surgery by the certified surgeon becomes possible.
Required Documents
- Copies of da Vinci System Training completion certificates for all certified surgeons (assistants not required) (issued by Intuitive Surgical)
- Copies of documents certifying completion of case observation and laboratory training using cadavers (issued by the training facility)
Implementing Facility Certification Application Form [PDF file 26KB]
Certified facilities must pay an annual membership fee (50,000 yen) as a corporate facility member.
The annual membership fee is on a fiscal year basis (April 1 to March 31 of the following year).
If facility membership is declined, the facility certification will be revoked.